Developments in possible pandemic prevention are currently making the news. This week, drug manufacturer Glaxo Smith-Kline became the first to be granted approval of its pre-pandemic influenza vaccine. The vaccine, called Prepandrix, uses the current H5N1 influenza virus, otherwise known as bird flu. This strain, which to date has affected birds and humans who live and work closely with birds, has caused much concern, over its possible mutation to allow human to human transmission. Health officials have repeatedly warned that if this happens, a bird flu pandemic is highly likely. The European Commission has approved Prepandrix for use in all 27 EU member states.
Smith-Kline became the first to be granted approval of its pre-pandemic influenza vaccine. The vaccine, called Prepandrix, uses the current H5N1 influenza virus, otherwise known as bird flu. This strain, which to date has affected birds and humans who live and work closely with birds, has caused much concern, over its possible mutation to allow human to human transmission. Health officials have repeatedly warned that if this happens, a bird flu pandemic is highly likely. The European Commission has approved Prepandrix for use in all 27 EU member states.
So how will the new vaccine work? Like a typical flu jab, it will be given as a preventative measure. The vaccine, containing a blended dose of existing H5N1 strains from Vietnam and Indonesia, will ‘teach’ the human immune system to recognise the virus and fight it off. It is apparently flexible, so should still prove effective if the virus mutates slightly. GSK has donated 50 million doses of Prepandrix to the World Health Organisation, and will be offering it at a “much reduced” price to all developing nations.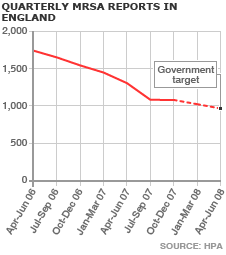
Meanwhile, MRSA, the hospital superbug, has also been in the headlines. A British company called Destiny Pharma has developed a drug, codenamed XF-73, which has proved effective against the bacteria in initial trials. The drug is placed as a gel in the nose, interacting lethally with the bacteria’s cell membrane, giving bugs less opportunity to develop and infiltrate a patient’s bloodstream. However, the latest figures from the Health protection Agency show that drops in the number of MRSA infections appear to have stalled.
You can read up on the facts and stats behind bird flu, MRSA and how pandemics spread in our pocket guide – Pocket Issue, Pandemics: Bird flu, MRSA – do we need to worry?
